- Covalent Bonds Lewis Structures Worksheet
- Lewis Dot Structure Covalent Bonds Calculator Present Value
Illustrate covalent bond formation with Lewis electron dot diagrams. Draw Lewis structures depicting the bonding in simple molecules Ionic bonding typically occurs when it is easy for one atom to lose one or more electrons and another atom to gain one or more electrons. Chlorine has 17 electrons, but 10 of those are in the orbitals of the lower energy levels. (1S, 2S, 2P orbitals). These are completely enveloped by the larger 3S-orbital (think of a golfball inside a tennisball) so takes no part in the formation of bonds. The other seven are distributed in the 3S and the three 3P-orbitals, but upon forming (Covalent) bonds these orbitals.
Could someone explain the lewis structure diagram of covalent compound Al2Cl6?
Also, why is Al2Cl6 (aluminium chloride) covalent?
Here's what we have to know for school but I don't know how it works. What do the arrows mean?
Also, why is Al2Cl6 (aluminium chloride) covalent?
Here's what we have to know for school but I don't know how it works. What do the arrows mean?
1 Answer
Explanation:

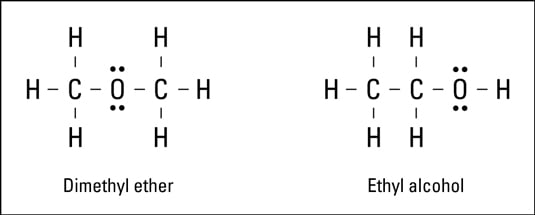
Chlorine has 17 electrons, but 10 of those are in the orbitals of the lower energy levels. (
These are completely enveloped by the larger
The other seven are distributed in the
for more info about Hybridisation, here's a good link:
In our case the
(Picture courtesy of https://en.wikipedia.org/wiki/Orbital_hybridisation)
Each orbital can contain, and indeed strives to contain, 2 electrons (
Chlorine thus has 7 electrons in the 4
The fourth one, the one that contains the single, unpaired
Aluminium has only 3 electrons: 2 in the
Chlorine has a rather high Electronegativity, which means that it pulls rather hard at electrons from other atoms (from each other, and from other elements.
It is a tug-of-war that the Aluminium atom is threatening to lose, leaving it rather
At the same time, the Chlorine atoms are satisfied in their hunger for electrons, in fact they don't want any more because they have a full set of 8!
Because of this 'diminished appetite' on the side of the Chlorine atoms, and the increased hunger on the part of the Aluminium atom, The bond is formed by BOTH of the Chlorine electrons.
This is the explanation for the arrow circled by Aqua:
In
Covalent Bonds Lewis Structures Worksheet


Hope this helps...
PS: By the way, it is covalent because the bonds are created by sharing of electrons between the two atoms.